
Forxiga: New indication approved
Forxiga, known generically as Dapagliflozin, is a sodium-glucose co-transporter 2 inhibitor (SGLT2i) developed and marketed by AstraZeneca. SGLT2 inhibitors act by reducing renal glucose reabsorption, leading to increased urinary glucose excretion and, consequently, lower blood glucose levels.
Clinical milestones and expanded indications beyond diabetes
| Trial | Underlying Condition of the cohort | Key Finding/Indication |
| DECLARE-TIMI 58 (2018) Cardiovascular trial | Type 2 diabetes | Reduced risk of cardiovascular death or hospitalisation for heart failure |
| DAPA-HF Heart failure (2019) | Symptomatic heart failure with reduced ejection fraction (HFrEF) | Expanded indication for Dapagliflozin in adults |
| DAPA-CKD (2020) | Chronic kidney disease | Approved to reduce the risk of sustained decline in kidney function, end-stage kidney disease, and cardiovascular death |
| Pharmaceutical Benefits Scheme (PBS) Approvals Dapagliflozin | |||
| Type 2 Diabetes | HbA1c > 7% on Metformin or Sulfonylurea (not with GLP-1 agonist) | ||
| Heart Failure | Chronic left ventricular heart failure regardless of diabetes (symptomatic with NYHA classes II, III or IV) | ||
| Chronic kidney disease | Chronic Kidney disease, regardless of diabetes | ||
| Expanded Type 2 Diabetes Access | Regardless of HbA1c with Metformin, unless not tolerated with: High risk of a cardiovascular event or have cardiovascular disease or identify as an Aboriginal or Torres Strait Islander. This is regardless of HbA1c. The combination product Xigduo XR. (not with GLP-1 agonist) | ||
Early intervention for people with diabetes and cardiovascular conditions
The new indication enables subsidised early intervention for people with diabetes and cardiovascular conditions, or those at high risk, including Aboriginal and Torres Strait Islander individuals. This access is regardless of a person’s current glucose levels. Previously, eligibility required an HbA1c level greater than 7% and the use of Metformin. While Metformin requirements remain, people with current cardiovascular conditions or who identify as Aboriginal and Torres Strait Islander no longer need to demonstrate hyperglycaemia.
Aboriginal and Torres Strait Islander people are specifically included because they face higher cardiovascular risks with diabetes. After adjusting for age, cardiovascular disease death rates among Indigenous Australians are 1.5 times higher than non-Indigenous Australians, with earlier and more frequent hospitalisations. (2)
Combined Dapagliflozin and Metformin tablet
The combination tablet Xigduo, containing Dapagliflozin and Metformin (extended release), improves ease and frequency of use, thereby reducing the potential adverse effects of Metformin.
Figure 2 below presents a simulated pharmacokinetic comparison of formulations of the same drug, illustrating the differences between immediate- and extended-release properties. With once-daily dosing of an extended-release medication with a short half-life, blood levels are more often in the therapeutic zone and less often create adverse effects.
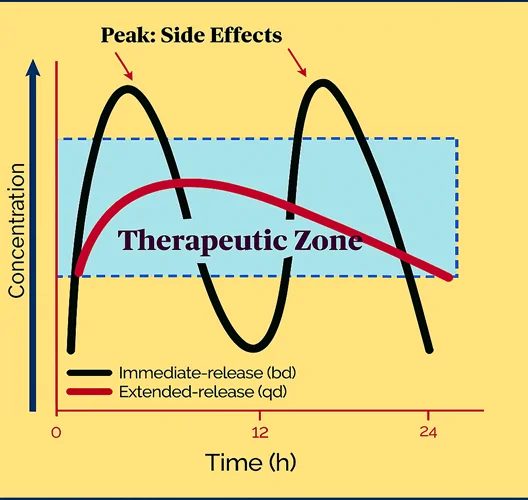
Figure 2: Simulated pharmacokinetic comparison of blood levels resulting from formulations of the same drug, immediate release (twice a day)and extended release (once a day).
People using chronic medications with once-daily dosing regimens showed an estimated 39-61% higher adherence rate compared to those on twice-daily dosing regimens. (3)
Summary
In summary, these changes represent a significant advancement in the management of chronic kidney disease and type 2 diabetes, particularly for those at elevated cardiovascular risk. By broadening access to early intervention therapies and addressing structural barriers for high-risk groups, including Aboriginal and Torres Strait Islander people, these updates offer the potential to improve health outcomes and reduce longstanding disparities in care.
References
1. DAPA-CKD: The Beginning of a New Era in Renal Protection. David Z.I. Cherney, Subodh Verma. 1, 2021, JACC: Basic to Translational Science, Vol. 6, pp. 74-77.
2. Australian Government, Australian Institute of Health and Welfare, National Indigenous Australians Agency. Tier 1 – Health Status and Outcomes: 1.05 Cardiovascular disease. Aboriginal and Torres Strait Islander, Health Performance Framework. [Online] [Cited: 9th September 2025.] https://www.indigenoushpf.gov.au/measures/1-05-cardiovascular-disease.
3. Impact of once-daily versus twice-daily dosing frequency on adherence to chronic medications among patients with venous thromboembolism. Laliberté F, Bookhart BK, Nelson WW, Lefebvre P, Schein JR, Rondeau-Leclaire J, Duh MS. 3, 2013, Patient, Vol. 6, pp. 213-24.
By Donna Itzstein, Pharmacist and Credentialled Diabetes Educator
